10/26/15 – ClearPrep Obtains TGA approval in Australia
ClearPrep Obtains TGA approval in Australia Australian approval also opens the door in New Zealand. TUSTIN, CALIFORNIA – ClearPrep Next-Generation Liquid-Based Cytology has been approved by the Therapeutic Goods Administration […]
10/20/15 – ClearPrep Obtains ANVISA approval in Brazil
ClearPrep Obtains ANVISA approval in Brazil TUSTIN, CALIFORNIA – Resolution Biomedical, Inc. announced today that its ClearPrep Next- Generation Liquid-Based Cytology products have been approved by the Brazilian Health Surveillance […]
5/27/15 – Resolution Biomedical Granted US Patent
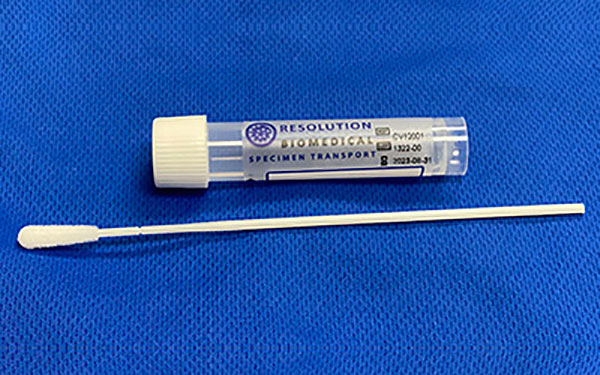
Resolution Biomedical Granted US Patent Unique collection vial improves celluarity of collected sample. TUSTIN, CALIFORNIA – Resolution Biomedical, Inc. announced today that it has been issued US Patent No. 9,039,636, […]
1/21/15 – Resolution Biomedical Obtains ISO 13485 Certification
Resolution Biomedical Obtains ISO 13485 Certification Internationally-recognized standard opens the door to registration in multiple countries. TUSTIN, CALIFORNIA – Resolution Biomedical, Inc. announced today that it has received ISO 13485 […]
2/27/14 – Forward Science Technologies & Resolution Biomedical Partner to Launch Liquid-Cytology Testing for Dental Industry
Forward Science Technologies & Resolution Biomedical Partner to Launch Liquid-Cytology Testing for Dental Industry Forward Science Technologies is the first American company to incorporate fluorescence technology and liquid-based cytology to […]